Understanding Periodic Trends and Their Importance
Related Articles: Understanding Periodic Trends and Their Importance
Introduction
With great pleasure, we will explore the intriguing topic related to Understanding Periodic Trends and Their Importance. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Understanding Periodic Trends and Their Importance
- 2 Introduction
- 3 Understanding Periodic Trends and Their Importance
- 3.1 Key Periodic Trends
- 3.2 Importance of Periodic Trends
- 3.3 Periodic Trends Worksheet Answer Key 2025
- 3.3.1 Why is the Periodic Trends Worksheet Answer Key 2025 Important?
- 3.3.2 How to Use the Periodic Trends Worksheet Answer Key 2025 Effectively
- 3.4 Related Searches
- 3.4.3 1. Periodic Trends Worksheet PDF:
- 3.4.4 2. Periodic Trends Worksheet Answers:
- 3.4.5 3. Periodic Trends Worksheet High School:
- 3.4.6 4. Periodic Trends Worksheet AP Chemistry:
- 3.4.7 5. Periodic Trends Worksheet College:
- 3.4.8 6. Periodic Trends Worksheet with Answers:
- 3.4.9 7. Periodic Trends Worksheet Free:
- 3.4.10 8. Periodic Trends Worksheet for Dummies:
- 3.5 FAQs
- 3.6 Tips for Mastering Periodic Trends
- 3.7 Conclusion
- 4 Closure
Understanding Periodic Trends and Their Importance
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
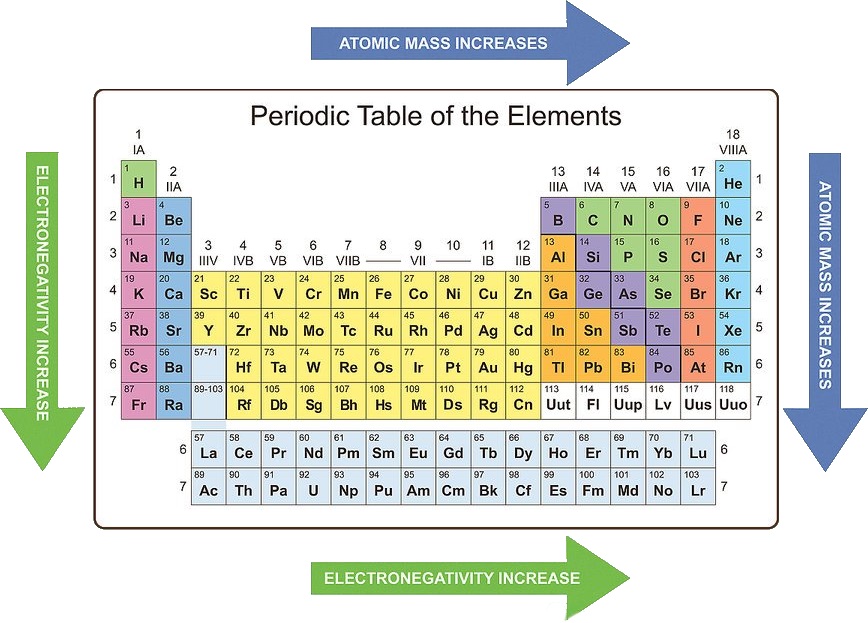
The periodic table, a fundamental tool in chemistry, organizes elements based on their atomic structure and recurring properties. Periodic trends are the predictable patterns in these properties as you move across a period (horizontal row) or down a group (vertical column) of the periodic table. Understanding these trends is crucial for comprehending chemical behavior and predicting reactions.
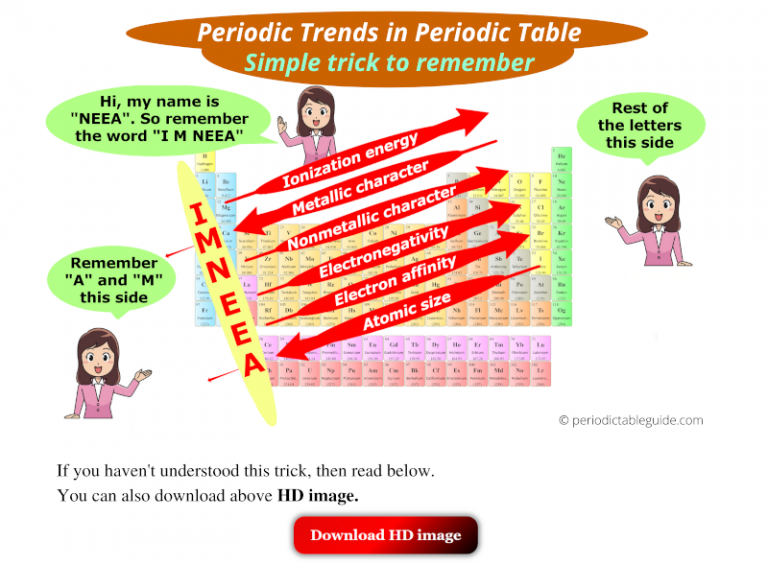
Key Periodic Trends
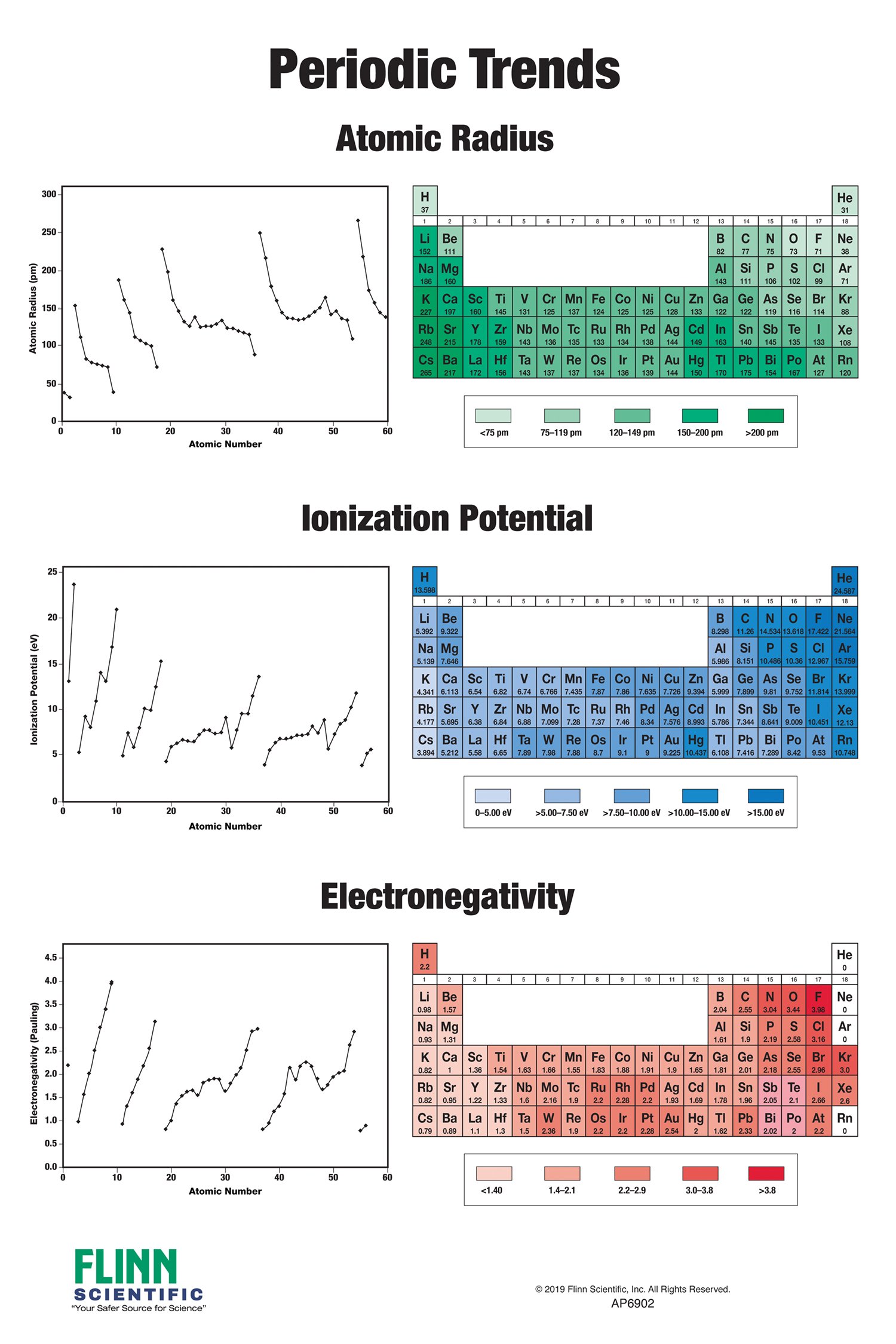
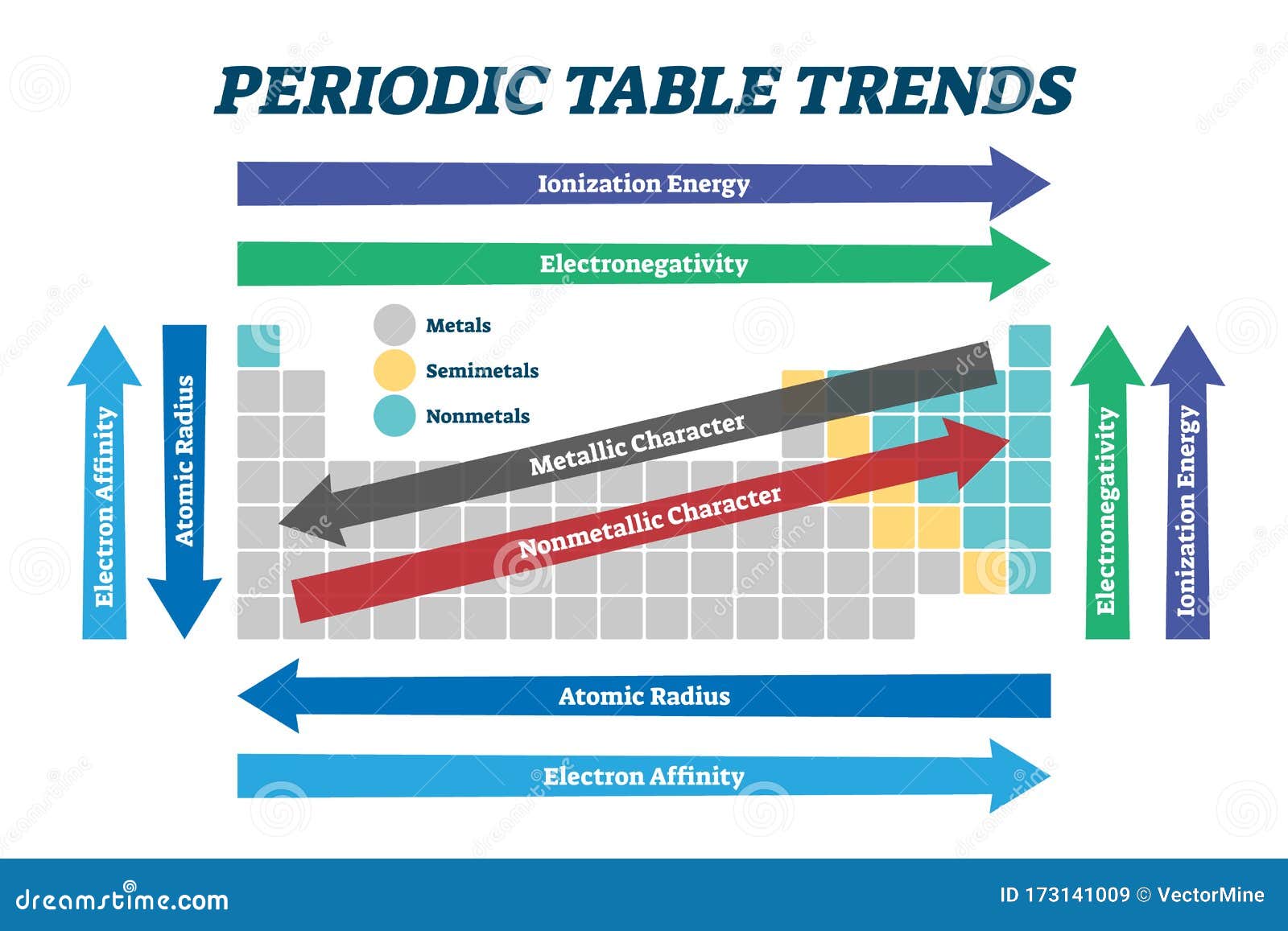
1. Atomic Radius: The atomic radius is the distance from the nucleus to the outermost electron shell.
- Across a period: Atomic radius decreases. This is because the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons, pulling them closer.
- Down a group: Atomic radius increases. The addition of electron shells with increasing principal quantum numbers (n) pushes the outer electrons further away from the nucleus.
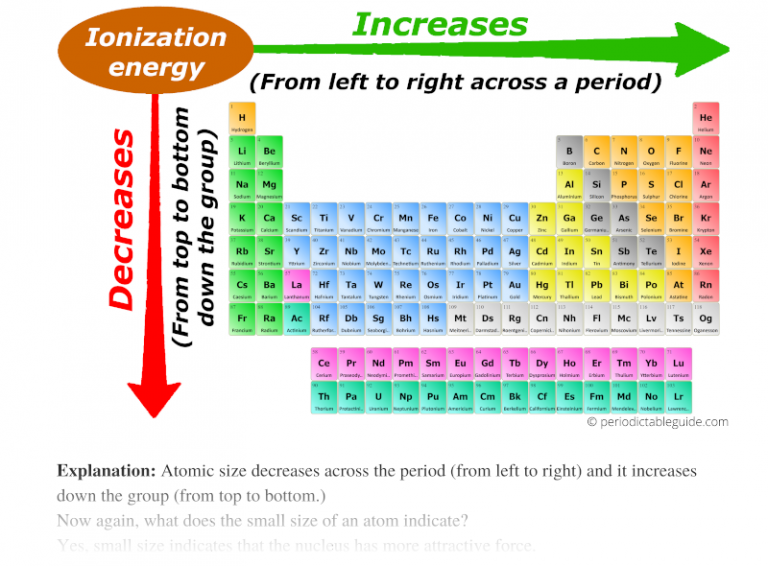
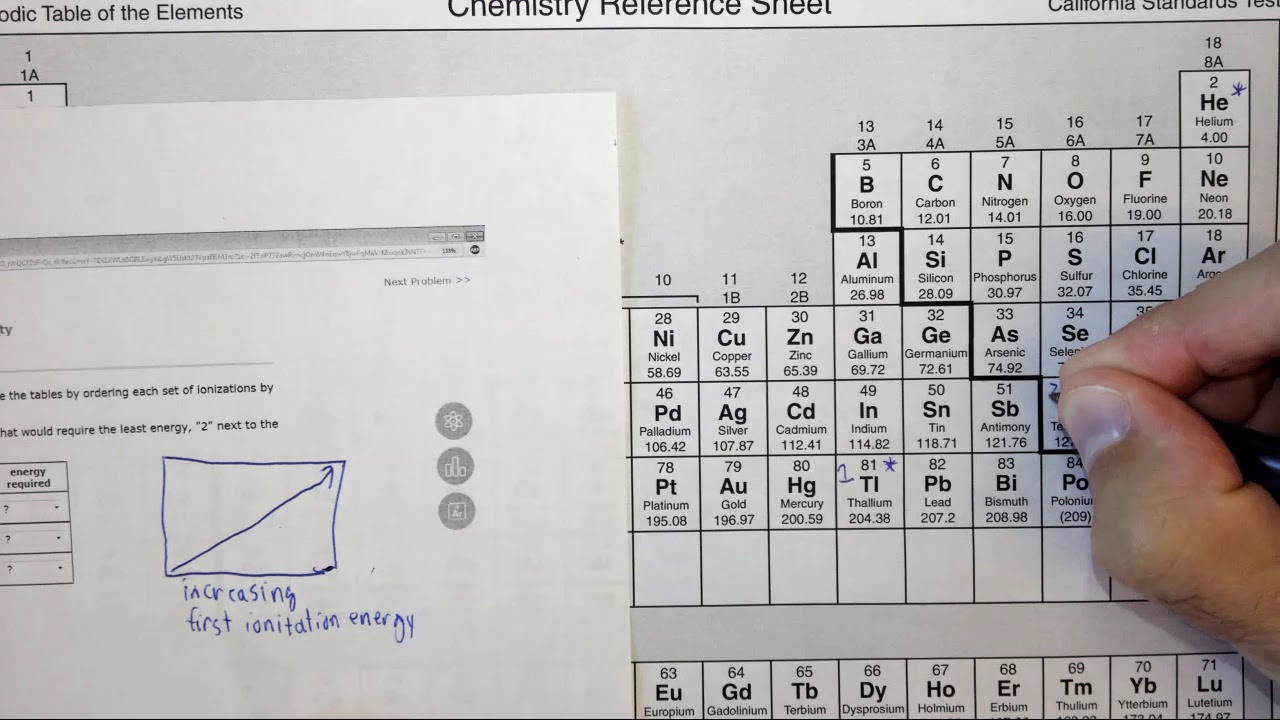
2. Ionization Energy: Ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground state.
- Across a period: Ionization energy increases. The increasing nuclear charge across a period leads to a stronger hold on the electrons, requiring more energy to remove them.
- Down a group: Ionization energy decreases. The outer electrons are further from the nucleus, experiencing weaker attraction and requiring less energy to remove.
3. Electron Affinity: Electron affinity is the change in energy when an electron is added to a neutral atom in its gaseous state.
- Across a period: Electron affinity generally increases. The attraction of the nucleus for electrons increases, making it more favorable to add an electron. However, there are exceptions, like the noble gases, which have a very low electron affinity.
- Down a group: Electron affinity generally decreases. The outer electron shells are further from the nucleus, experiencing weaker attraction and making the addition of an electron less favorable.
4. Electronegativity: Electronegativity is the ability of an atom in a molecule to attract electrons towards itself.
- Across a period: Electronegativity increases. The increasing nuclear charge attracts electrons more strongly.
- Down a group: Electronegativity decreases. The outer electrons are further from the nucleus, experiencing weaker attraction and making the atom less electronegative.
5. Metallic Character: Metallic character refers to the tendency of an element to lose electrons and form positive ions.
- Across a period: Metallic character decreases. Elements become more non-metallic as the tendency to gain electrons increases.
- Down a group: Metallic character increases. Elements become more metallic as the tendency to lose electrons increases due to the larger atomic size and weaker hold on the outer electrons.
Importance of Periodic Trends
Understanding periodic trends is crucial for several reasons:
- Predicting Chemical Behavior: Periodic trends allow us to predict how elements will behave in chemical reactions. For instance, knowing that electronegativity increases across a period helps us understand why elements like fluorine and oxygen are highly reactive, readily attracting electrons from other elements.
- Understanding Chemical Bonding: Trends like electronegativity and ionization energy are essential for understanding the formation of chemical bonds. For example, the difference in electronegativity between two atoms determines the type of bond they will form (ionic, covalent, or polar covalent).
- Designing New Materials: Knowledge of periodic trends is critical in designing new materials with specific properties. For example, understanding the relationship between atomic radius and melting point can help us predict the melting point of a new alloy.
- Interpreting Experimental Results: Periodic trends provide a framework for interpreting experimental results. For instance, observing a higher ionization energy for an element than expected can indicate the presence of a full or half-filled electron shell.
Periodic Trends Worksheet Answer Key 2025
The periodic trends worksheet answer key 2025 is a valuable resource for students studying chemistry. It provides solutions to various exercises that test their understanding of periodic trends. This key can be used for self-assessment, helping students identify areas where they need further practice and clarification.
Why is the Periodic Trends Worksheet Answer Key 2025 Important?
- Reinforces Learning: The answer key provides a clear and concise explanation of the correct answers, reinforcing the concepts learned in class.
- Identifies Gaps in Understanding: By comparing their answers with the key, students can identify areas where they have made mistakes, highlighting gaps in their understanding.
- Offers Guidance for Improvement: The key can guide students towards specific topics or concepts they need to revisit and practice further.
- Encourages Independent Learning: The answer key empowers students to take ownership of their learning by allowing them to check their work and identify areas for improvement.
How to Use the Periodic Trends Worksheet Answer Key 2025 Effectively
- Complete the Worksheet First: It is essential to attempt the worksheet independently before referring to the answer key. This allows students to engage with the concepts and test their understanding.
- Compare Answers Carefully: Carefully compare your answers with the key, noting any discrepancies.
- Analyze Mistakes: For incorrect answers, try to understand why you made the mistake. Was it a misunderstanding of the concept, a calculation error, or a simple oversight?
- Revisit Relevant Material: If you identify gaps in your understanding, revisit the relevant sections in your textbook or class notes.
- Seek Clarification: If you are still struggling with a concept, do not hesitate to seek clarification from your teacher or a tutor.
Related Searches
1. Periodic Trends Worksheet PDF:
Many online resources offer printable periodic trends worksheets in PDF format. These worksheets typically cover various aspects of periodic trends, including atomic radius, ionization energy, electronegativity, and metallic character. They often include practice problems and diagrams to help students visualize the concepts.
2. Periodic Trends Worksheet Answers:
Several websites provide answers to specific periodic trends worksheets. These answers can be valuable for students who are struggling with a particular concept or need to verify their solutions.
3. Periodic Trends Worksheet High School:
High school chemistry courses often include extensive coverage of periodic trends. Worksheets designed for high school students typically delve into the concepts in greater depth and may include more challenging problems.
4. Periodic Trends Worksheet AP Chemistry:
AP Chemistry courses require a deeper understanding of periodic trends and their applications. Worksheets designed for AP Chemistry students may include more complex problems and require a higher level of critical thinking.
5. Periodic Trends Worksheet College:
College-level chemistry courses often cover periodic trends in greater detail, exploring their implications in various fields like organic chemistry and inorganic chemistry. Worksheets designed for college students may include more advanced concepts and applications.
6. Periodic Trends Worksheet with Answers:
Some worksheets include answer keys within the document, making it easier for students to check their work and identify areas for improvement.
7. Periodic Trends Worksheet Free:
Many free resources are available online, including periodic trends worksheets and their answer keys. These resources can be valuable for students who are looking for additional practice or need help with specific concepts.
8. Periodic Trends Worksheet for Dummies:
"For Dummies" guides often provide simplified explanations of complex concepts, making them accessible to a wider audience. Periodic trends worksheets for dummies can be helpful for students who are new to the topic or struggling to grasp the concepts.
FAQs
Q: What are some common mistakes students make when working with periodic trends?
A: Students often make mistakes when applying the trends, confusing the direction of change across a period and down a group. For example, they might incorrectly state that atomic radius increases across a period. Another common mistake is failing to recognize exceptions to the general trends, such as the electron affinity of noble gases.
Q: How can I make learning periodic trends more engaging?
A: Engaging with periodic trends can involve using visual aids, like diagrams and animations, to illustrate the concepts. Creating flashcards or mind maps can help with memorization. You can also try to relate the concepts to real-world applications, like the use of different metals in everyday objects.
Q: What are some resources for further learning about periodic trends?
A: Several resources can help you delve deeper into periodic trends. Textbooks, online tutorials, and videos are excellent options. You can also explore websites like Khan Academy and ChemWiki for interactive lessons and practice problems.
Tips for Mastering Periodic Trends
- Focus on the Core Concepts: Understand the fundamental principles behind each trend, such as the role of nuclear charge and electron shielding.
- Use Visual Aids: Diagrams, animations, and interactive simulations can help you visualize the trends and make them easier to understand.
- Practice, Practice, Practice: Work through various practice problems and worksheets to solidify your understanding and apply the concepts.
- Relate to Real-World Examples: Try to connect periodic trends to real-world applications, such as the properties of different metals or the reactivity of elements in everyday chemicals.
- Seek Help When Needed: Don’t hesitate to ask your teacher, a tutor, or a classmate for help if you are struggling with a concept.
Conclusion
Periodic trends are fundamental to understanding the behavior of elements and predicting their reactions. By mastering these trends, you gain a powerful tool for interpreting chemical phenomena and exploring the fascinating world of chemistry. The periodic trends worksheet answer key 2025 can be a valuable resource in your journey of learning and mastering these concepts. Remember to use it effectively, practice diligently, and seek help when needed to achieve a deeper understanding of the periodic table and its intricate patterns.
.PNG)

Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends and Their Importance. We thank you for taking the time to read this article. See you in our next article!