Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025
Related Articles: Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025
Introduction
With great pleasure, we will explore the intriguing topic related to Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025
- 2 Introduction
- 3 Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025
- 3.1 Understanding Bond Dissociation Energy
- 3.2 Bond Dissociation Energy Trends in 2025:
- 3.3 Related Searches: Bond Dissociation Energy Trends in 2025
- 3.4 FAQs: Bond Dissociation Energy Trends in 2025
- 3.5 Tips: Bond Dissociation Energy Trends in 2025
- 3.6 Conclusion: Bond Dissociation Energy Trends in 2025
- 4 Closure
Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025

The realm of chemistry hinges upon the intricate dance of atoms, their interactions forming the building blocks of all matter. One crucial aspect of this dance is the strength of the bonds that hold atoms together, a measure known as bond dissociation energy. This article delves into the anticipated trends in bond dissociation energy in 2025, exploring its significance in various fields and highlighting its potential impact on scientific advancements.
Understanding Bond Dissociation Energy
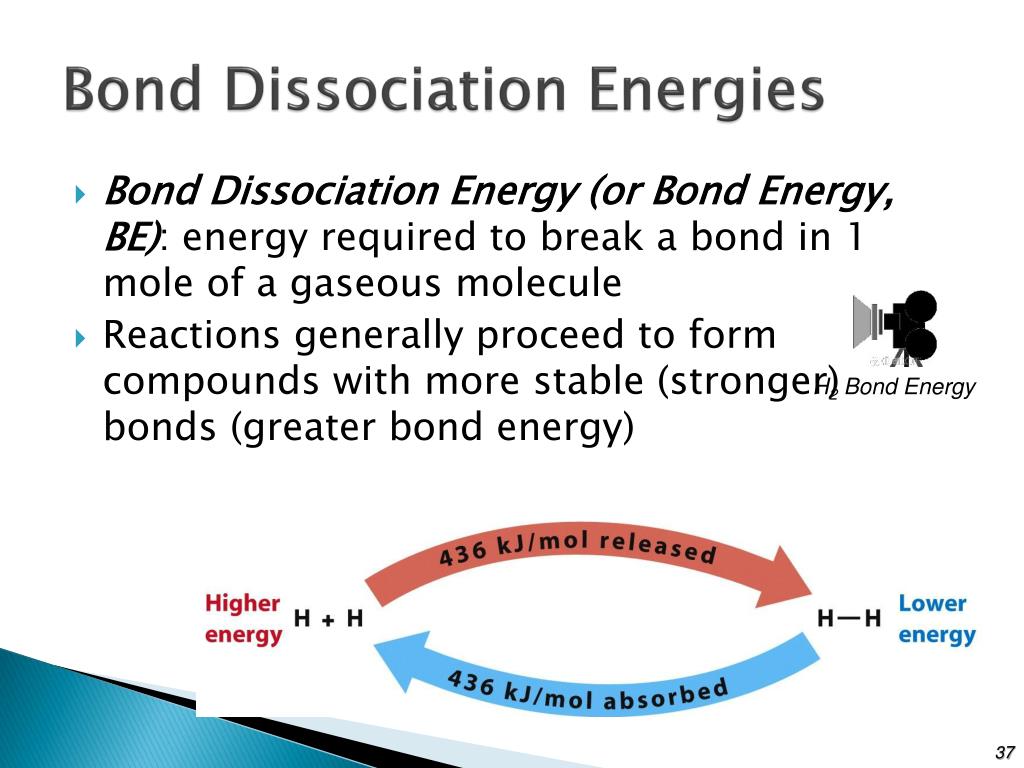
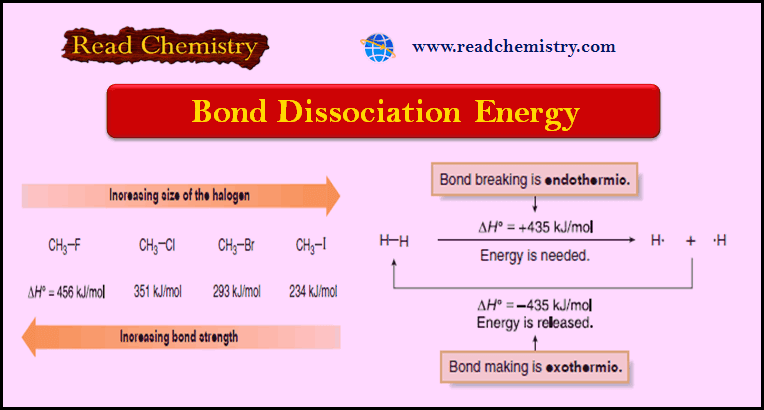
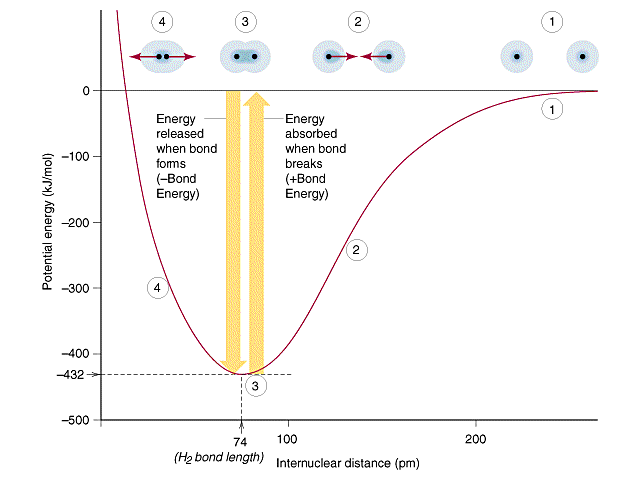
Bond dissociation energy (BDE) refers to the amount of energy required to break a specific chemical bond in a molecule, homolytically, forming two radicals. This energy is expressed in units of kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).
Factors Influencing Bond Dissociation Energy:
Several factors contribute to the strength or weakness of a chemical bond, influencing its BDE:
- Bond Order: A higher bond order (the number of chemical bonds between two atoms) generally corresponds to a stronger bond and higher BDE. For instance, a triple bond between carbon atoms is stronger than a double bond or a single bond.
- Bond Length: Shorter bond lengths imply stronger attractions between atoms, resulting in higher BDE.
- Electronegativity: The difference in electronegativity between atoms involved in a bond influences its polarity and strength. Polar bonds tend to be stronger than non-polar bonds.
- Hybridization: The hybridization of atomic orbitals involved in bonding significantly impacts the BDE. For example, sp3 hybridized orbitals in single bonds generally have lower BDE compared to sp2 hybridized orbitals in double bonds.
- Steric Effects: The spatial arrangement of atoms and groups around the bond can influence its strength due to steric hindrance.
Bond Dissociation Energy Trends in 2025:
Predicting precise BDE values for specific molecules in 2025 is challenging due to the dynamic nature of scientific research. However, several trends are likely to shape the landscape of bond dissociation energy research and applications:
1. Advancements in Computational Chemistry:
Computational chemistry methods, particularly density functional theory (DFT) calculations, are becoming increasingly sophisticated. This enables more accurate predictions of BDE values for complex molecules, even those not yet synthesized. Researchers can utilize these methods to:
- Design novel molecules with specific bond strengths: This allows for the development of materials with tailored properties, such as high thermal stability or enhanced reactivity.
- Explore the mechanisms of chemical reactions: Understanding BDE values helps elucidate the energy barriers and transition states involved in chemical reactions, paving the way for more efficient and controlled processes.
2. Focus on Heterocyclic Chemistry:
Heterocyclic compounds, containing atoms other than carbon in their ring structures, are increasingly prominent in pharmaceutical, agricultural, and materials science. Understanding the BDEs of bonds within these complex molecules is critical for:
- Developing new drugs and pesticides: BDEs can guide the design of molecules with specific pharmacological activity or pesticide efficacy.
- Improving the synthesis of heterocyclic materials: Understanding the strength of bonds within heterocyclic rings allows for optimizing synthetic routes and minimizing side reactions.
3. Emerging Applications in Materials Science:
- Polymer Science: BDE values are crucial for understanding the degradation and stability of polymers. Predicting and manipulating BDEs can lead to the development of polymers with enhanced durability and resistance to environmental degradation.
- Nanomaterials: The unique properties of nanomaterials often arise from their surface chemistry and bond strengths. Understanding and manipulating BDEs at the nanoscale can lead to the development of advanced materials with tailored electronic, optical, and catalytic properties.
4. Impact on Green Chemistry:
The quest for sustainable and environmentally friendly chemical processes is driving research into bond dissociation energy. Understanding BDEs can help:
- Develop more efficient catalysts: By understanding the bond strengths involved in catalytic reactions, researchers can design catalysts that promote desired reactions while minimizing energy consumption and waste generation.
- Design safer and more environmentally benign chemicals: BDEs can guide the development of chemicals with reduced toxicity and improved biodegradability.
Related Searches: Bond Dissociation Energy Trends in 2025
1. Bond Dissociation Energy Calculations: Exploring the computational methods employed for calculating BDE values, including DFT, ab initio methods, and semi-empirical methods.
2. Bond Dissociation Energy Database: Investigating the availability and accessibility of comprehensive databases containing experimentally determined and computationally predicted BDE values for various molecules.
3. Bond Dissociation Energy and Reaction Mechanisms: Examining the role of BDE in understanding and predicting the mechanisms of chemical reactions, including bond breaking, bond formation, and radical reactions.
4. Bond Dissociation Energy and Polymer Stability: Delving into the relationship between BDE and the degradation and stability of polymers, exploring the influence of bond strengths on polymer properties and lifespan.
5. Bond Dissociation Energy and Nanomaterials: Analyzing the significance of BDE in characterizing the surface chemistry and reactivity of nanomaterials, highlighting its role in tailoring the properties of these materials.
6. Bond Dissociation Energy and Green Chemistry: Exploring the application of BDE principles in developing sustainable and environmentally friendly chemical processes, focusing on catalyst design and the development of safer chemicals.
7. Bond Dissociation Energy and Medicinal Chemistry: Examining the role of BDE in drug discovery and development, particularly in understanding the interactions between drugs and their target molecules.
8. Bond Dissociation Energy and Atmospheric Chemistry: Investigating the influence of BDE on the stability and reactivity of atmospheric molecules, including ozone depletion and the formation of pollutants.
FAQs: Bond Dissociation Energy Trends in 2025
1. How is Bond Dissociation Energy Measured?
Bond dissociation energy can be determined experimentally through various techniques, including:
- Spectroscopic methods: Techniques like ultraviolet-visible (UV-Vis) spectroscopy and infrared (IR) spectroscopy can be used to study the absorption and emission of energy by molecules, providing insights into bond strengths.
- Mass spectrometry: Mass spectrometry analyzes the fragmentation patterns of molecules under controlled conditions, providing information about bond energies.
- Kinetic studies: By measuring the rate of chemical reactions, researchers can determine the activation energy required to break specific bonds, which is related to BDE.
2. What is the Significance of Bond Dissociation Energy in Chemistry?
BDE is a fundamental concept in chemistry, providing insights into:
- The stability of molecules: Higher BDE values indicate stronger bonds and more stable molecules.
- The reactivity of molecules: Weaker bonds with lower BDE values are more susceptible to breaking, leading to higher reactivity.
- The mechanisms of chemical reactions: BDE values help elucidate the energy barriers and transition states involved in chemical reactions.
3. How Can Bond Dissociation Energy Be Used to Design New Materials?
Understanding BDE allows for the design of materials with tailored properties:
- High thermal stability: Materials with strong bonds and high BDE values exhibit greater resistance to heat and degradation.
- Enhanced reactivity: Materials with weaker bonds and lower BDE values can be designed for specific applications requiring reactivity, such as catalysts or polymers for controlled degradation.
4. What are the Challenges in Predicting Bond Dissociation Energy?
Predicting BDE values accurately can be challenging due to:
- Complexity of molecules: Complex molecules with multiple bonds and functional groups require sophisticated computational methods for accurate BDE predictions.
- Environmental factors: The presence of solvents, temperature, and other environmental factors can influence BDE values.
- Limitations of computational methods: Current computational methods have limitations in accurately predicting BDE values for all molecules and under all conditions.
Tips: Bond Dissociation Energy Trends in 2025
1. Stay Updated on Research: Keep abreast of the latest advancements in computational chemistry, particularly DFT methods, which are constantly improving their accuracy and applicability.
2. Explore Databases and Resources: Utilize available databases and online resources to access experimentally determined and computationally predicted BDE values for various molecules.
3. Apply BDE Principles in Research: Integrate the understanding of BDE into research projects, particularly in fields like materials science, drug discovery, and green chemistry.
4. Consider the Impact of Environmental Factors: Recognize that BDE values can be influenced by environmental factors and adjust experimental conditions or computational models accordingly.
5. Collaborate with Experts: Collaborate with researchers specializing in computational chemistry, theoretical chemistry, and experimental techniques to enhance the accuracy and reliability of BDE predictions.
Conclusion: Bond Dissociation Energy Trends in 2025
Bond dissociation energy remains a cornerstone concept in chemistry, driving advancements in various fields. As computational methods continue to evolve and research into complex molecules intensifies, the understanding of BDE will become even more crucial. By harnessing the power of bond dissociation energy, scientists can unlock the potential for developing novel materials, designing more efficient chemical processes, and paving the way for a more sustainable future.





Closure
Thus, we hope this article has provided valuable insights into Unveiling the Dynamics of Chemical Bonds: A Look at Bond Dissociation Energy Trends in 2025. We hope you find this article informative and beneficial. See you in our next article!